Copper removal from wine by copolymer PVI/PVP: Effects on copper components and binders
Research background
Copper (Cu) is a metal ion commonly found in all wines, with concentrations usually less than 0.5 mg/L. Copper in wine may come from viticulture, including application of fungicides and absorption from the soil, or during wine production (including contamination and additions caused by winemakers). In fact, copper is often deliberately added to wine to suppress reducing odors, such as those caused by hydrogen sulfide, by forming non-volatile complexes with these adverse aroma compounds. Excess hydrogen sulfide can make the copper in wine form copper organic acid complex, this form of copper can ensure that the wine is not affected by the accumulation of hydrogen sulfide in the short term. For copper sulfide complexes left over from wine production, subsequent filtration (0.45μm or 0.20μm) often fails to remove them. Finally, excess copper in wine can cause copper fog, but this usually requires a much higher concentration of copper than is typically encountered in modern winemaking.
The aim of the study was to evaluate the removal effects of two different types of polyvinylimidazole/polyvinylpyrrolidone (PVI/PVP) on different forms of copper in wine and standard wine samples. Through preliminary analysis, the study provides a certain explanation for the removal efficiency of copper in white wine is higher than that in red wine. The copper form is then measured using previously validated electrochemical, colorimetric and deep filtration techniques. To gain insight into whether copper binders remained in PVI/PVP treated wines, the study measured residual hydrogen sulfide and methylmercaptan concentrations and assessed the ability of treated wines to rebind copper. Finally, standard wine samples were studied to evaluate the removal activity of PVI/PVP on the clarified by-product of copper, i.e., polythiane.
Materials and methods
1 Chemical and wine samples
Cu (1000mg/L, ICP-grade), sodium sulfide (99.5%), sodium methsulfate (90%), L-cysteine (98%) and reduced L-glutathione (98%) were purchased from Sigma-Aldrich. The recycled Cellulose 0.2 μm Membrane Syringe Filter (Phenex) is provided by Phenomenex. Ultrapure water (18.2 MΩcm) is produced by the Milli-Q Plus purification system.
The 12%(v/v) ethanol aqueous solution used in this study consisted of 12%(v/v) ethanol aqueous solution, 0.011 M potassium hydrogen tartrate, and 0.007M tartaric acid (pH 3.2). The study used four white wines (pH 3.1±0.2, alcohol 12.0±0.5% (v/v)) and four red wines (pH 3.5±0.1, alcohol 13.0±0.5% (v/v)) from different grape varieties in 2019 and 2020. To provide multiple forms of copper in the wines studied, both white wines 4 (W4) and red wines 4 (R4) had 0.3 mg/L of copper (II) added prior to sample preparation, while no copper was added to the other wines.
2 PVI/PVP activity on copper
The following procedure Outlines the application of PVI/PVP in standard wines, white wines, and red wines, with experimental operations based primarily on standard commercial procedures for adding PVI/PVP to wines. The standard wine (3.5 L) was prepared using 12% (v/v) ethanol aqueous solution (pH 3.2) with 200 mg/L caffeic acid, 170 mg/L white wine polysaccharide and 50 mg/L sulfur dioxide. Polysaccharides are added to ensure that the standard wine behaves like white wine, and the smallest sulfide bound copper can be removed by adsorption on a regenerated cellulose membrane filter prior to instrumental analysis. The standard wine is degassed with nitrogen until the dissolved oxygen concentration is below 0.3 mg/L. It is then transferred to an anaerobic mask (oxygen concentration < 10% (v/v) air saturation) where all subsequent steps are carried out. This includes adding 129μg/L (3.78μM) of hydrogen sulfide (as sodium sulfide) and 0.3 mg/L (4.72μM) of copper (II) to the standard wine to give a molar ratio of 0.8:1 of hydrogen sulfide to copper (II). After 30 minutes, take 100 mL of the prepared standard wine sample and label it as “MW0” for analysis. The remaining standard wine is then completely filled with nine 250ml Schott bottles, which are sealed after treatment, leaving no headspace inside the bottle.
3 Activity of PVI/PVP against polythiane
The production of polythiane in standard wine is carried out as described by Bekker et al., with 8.5 mg/L hydrogen sulfide added to 200ml of standard wine. Take 100 ml of standard liquor solution, add 69 mg/L of L-cysteine, and add 181 mg/L of L-glutathione to the other 100 ml. Both samples were added with 5.6 mg/L iron (II) and 3.2 mg/L copper (II) and stored at room temperature for 72 hours without excluding any air. The sample was then prepared in triplicate and 10 mL of the prepared standard wine was transferred into a 22ml amber bottle and filled with nitrogen. Then 0.1 mL pre-prepared PVI/PVP suspension was added to obtain PVI/PVP with a concentration of 0.5 g/L, and 0.1 mL 12% (v/v) ethanol aqueous solution was added to the control sample. All samples were stirred at room temperature for 5 h and then filtered through a 0.2 μm RC filter for polythioane and total copper analysis.
4 Chemical Analysis
All copper analysis methods used previously validated methods (see Table 1). The total copper concentration in standard wine and red wine was analyzed by inductively coupled plasma emission spectrometry. Table 1 Outlines the different methods used to quantify copper content and its copper form in standard wine samples, white wines and red wines.

The gradient of the sample was (time, % A): 0 min, 97%; 10.0 min, 55%; 12.0 min, 25%; 17.5 min, 25%; 20.0 min,97%; 97% of 28 min. The injection volume was 5 μL, and the needle was washed with 10% (v/v) acetonitrile solution for 5 s between injections. The HRMS operates in extended dynamic range (2 GHz) mode with the positive ion mode set as follows: Scan rate 5 spectrum/SEC, reference ions for internal mass correction are purine (m/z 121.050873) and HP-0921 (m/z 922.009798), Dry gas temperature 300 ℃, dry gas flow rate 8 L/min, atomizer pressure 35 psi, sheath temperature 350℃, sheath gas flow rate 11 L/min, capillary voltage 5500 V, nozzle voltage 1000 V, shredder voltage 80 V, Separator 1 voltage 65 V and octopole RF peak voltage 750 V. The mass strength signal for each high-resolution m/z value corresponds to a different glutathione and cysteine-derived polythiane (Table 2) and is quantified as the equivalent of glutathione (m/z 308.091) and cysteine (m/z 122.027).

Research result
1 PVI/PVP total copper removal: white wine and red wine
In order to find out why the removal efficiency of total copper in PVI/PVP white wine is higher than that in red wine, the relationship between pH value and total copper removal was studied. The pH of wine is of particular interest, as it is known that red wines generally have a higher pH than white wines. Although the original study by Hirlam et al. did not publish the pH values of PVI/PVP treated wines, the study collected this dataset, and the results of the re-analysis of these data are shown in Figure 1.
As shown in Figure 1, a good relationship (R2 = 0.726) was established between the pH of the wine and the amount of copper removed (as a percentage of total copper), resulting in more efficient copper removal at lower pH of the wine. A similar result is obtained if absolute concentration units are used instead of the total copper removal rate (Figure 1).
It is speculated that at lower pH, PVI/PVP will be more favorable for competitive binding and/or adsorption of Cu, while at higher pH, the wine substrate can inhibit this copper removal mechanism. However, the data in Figure 1 deviated from a perfect linear fit, suggesting that factors other than the pH of the wine were also influencing the copper removal efficiency of PVI/PVP in these wine samples.
2 Measure the different copper components
Table 1 summarizes the different techniques for measuring copper content in standard, white and red wines, and also includes the main attribution forms for each component based on previous studies. In this study, the standard wine with hydrogen sulfide and copper (II) was analyzed by electrochemical method, and the sulfide-bound copper and sulfide-free copper in the standard wine could be distinguished. For white wine, three copper components were distinguished by colorimetric method, which mainly belong to copper organic acid, copper mercaptan and copper sulfide complex.
3 Effect of PVI/PVP treatment on total copper concentration and copper composition in wine
Figure 2 shows the concentration of total copper and other copper components in the wine before and after PVI/PVP treatment. A statistical analysis of the differences between total copper concentrations is included in Figure 2. In standard wines, white wines and red wines, the total copper concentration was significantly reduced after PVI/PVP treatment (Figure 2). According to the removal activity of PVI/PVP on different copper components, PVI/PVP can effectively reduce each copper component in white wine (Figure 2). The more efficient removal of copper from white wine compared to red wine is related to the pH of the wine (Figure 1), but this also seems to be a result of the fact that the copper component I is more easily removed from white wine than red wine (Figure 2).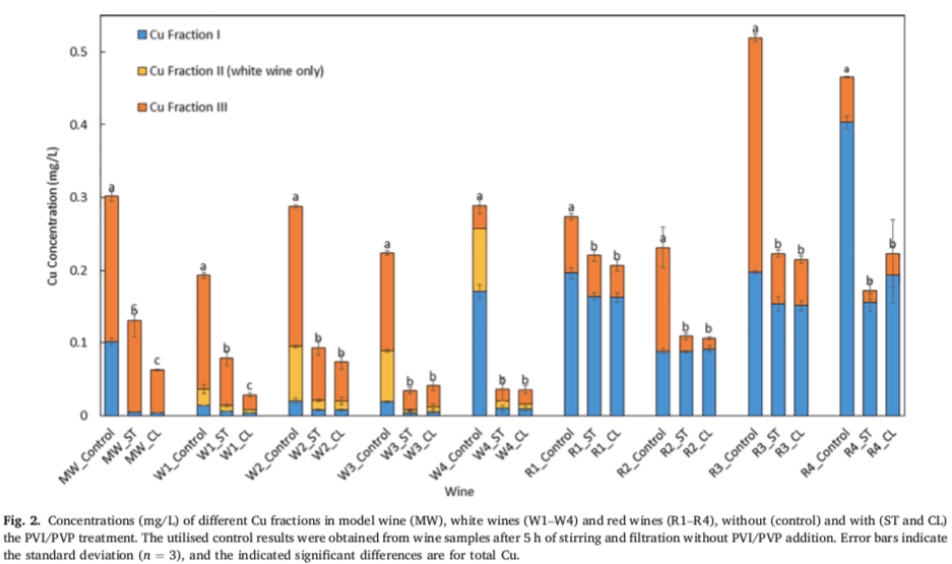
4. Influence of PVI/PVP on copper binder
Initially, the study evaluated the conversion capacity of PVI/PVP treated white wines to copper component III when 0.3 mg/L copper (II) was added. If PVI/ PVP separates copper from the copper sulfide complex, leaving the sulfide as uncomplexed hydrogen sulfide, the added copper (II) is readily converted to copper bound to the sulfide (i.e., Cu component III). In addition, if the copper sulfide complex is removed from PVI/PVP as a whole, the subsequent addition of copper (II) is mainly present in the copper organic acid and/or copper mercaptan complex in the form of copper components I and/or II. As shown in Figure 3, the copper (II) added to PVI/PVP treated white wine (W1-W4) can only form a trace amount of copper component III, which is mainly manifested as copper component I and copper component II. In fact, in all white wines treated with PVI/PVP, the amount of copper component III is considered negligible.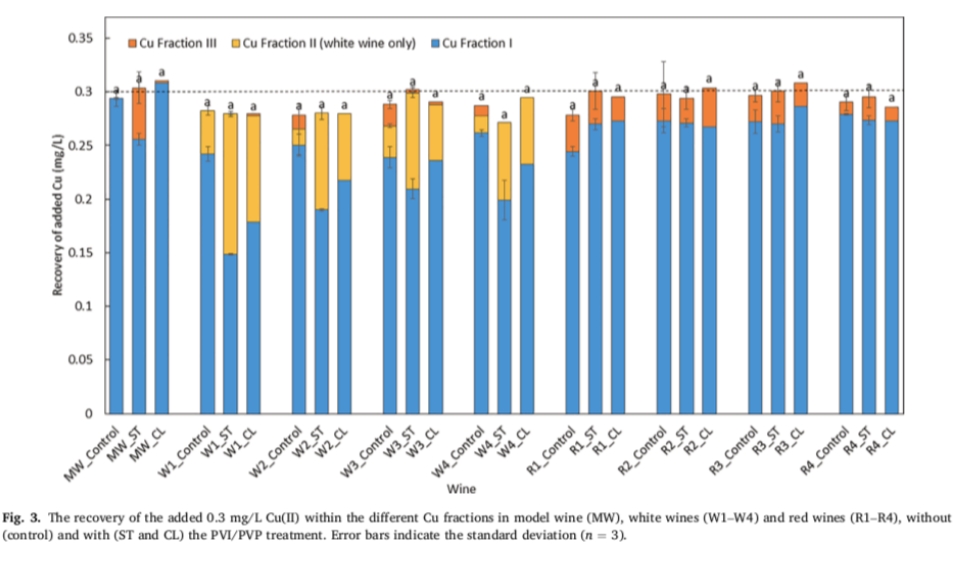
GC-SCD analysis of PVI/PVP treated and non-PVI /PVP treated wines also showed a reduction in the concentration of salt-releasing hydrogen sulfide in the wine after PVI/PVP treatment, further evidence that copper and its binding sulfide have been removed from the wine (Figure 4A). The proportion of balanced substances depends on the total mercaptan concentration and the concentration of copper components I and II in the wine. These results are also consistent with the absence of any reduction in methanethiol concentrations in wine before and after PVI/PVP treatment (Figure 4B).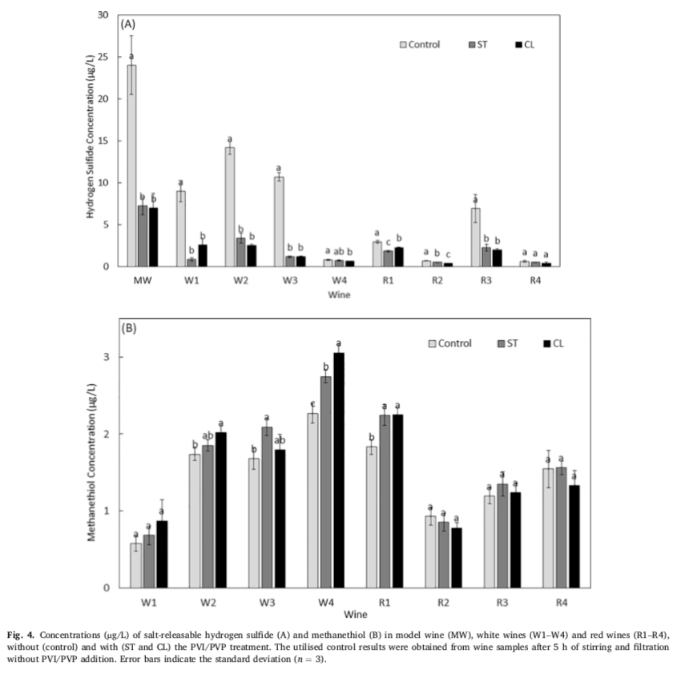
5 Effect of PVI/PVP treatment on polythioane concentration in wine
Once the copper, hydrogen sulfide, and mercaptan compounds in the wine react to form polythiols, it is unknown how strongly they associate or interact with copper and whether they can be removed from the wine by PVI/PVP treatment. As shown in Table 2, although copper has been effectively removed from this wine substrate, the concentration of polythiane is not affected by the PVI/PVP treatment of the standard wine. This result shows that even though PVI/PVP can effectively remove copper in the presence of polythioane, there is no strong interaction between PVI/PVP and copper, which means that the effect of PVI/PVP on the concentration of polythioane is negligible.
conclusion
The pH of the wine affects the removal of copper by PVI/PVP, so the removal of copper is more efficient at lower pH values. PVI/PVP treatment is effective in reducing all copper content in white wines, but in red wines, PVI/PVP is more efficient in removing copper bound to sulfides than copper bound to organic acids.
This result in red wine is most likely due to the higher pH of red wine which hinders the removal of organic acid-bound copper components. After PVI/PVP treatment of all wines, the sulfide has minimal binding effect on the added copper (II), meaning that the copper and sulfide in the copper complex bound to the sulfide are removed by PVI/PVP. For white wine, even though part of the copper bound to mercaptan is removed by copolymer PVI/PVP, the loss of mercaptan compounds after PVI/PVP treatment is small.
Although the copolymer effectively removed mercaptan associated copper, PVI/PVP had no effect on polythioane concentrations in standard wine samples. Of all forms of copper in wine, PVI/PVP has the highest affinity for copper associated with the formation of reducing aromas in wine and is able to remove copper and its harmful binders together.

